Urodiag®
Urine-based lab test for the surveillance of bladder cancer
Description
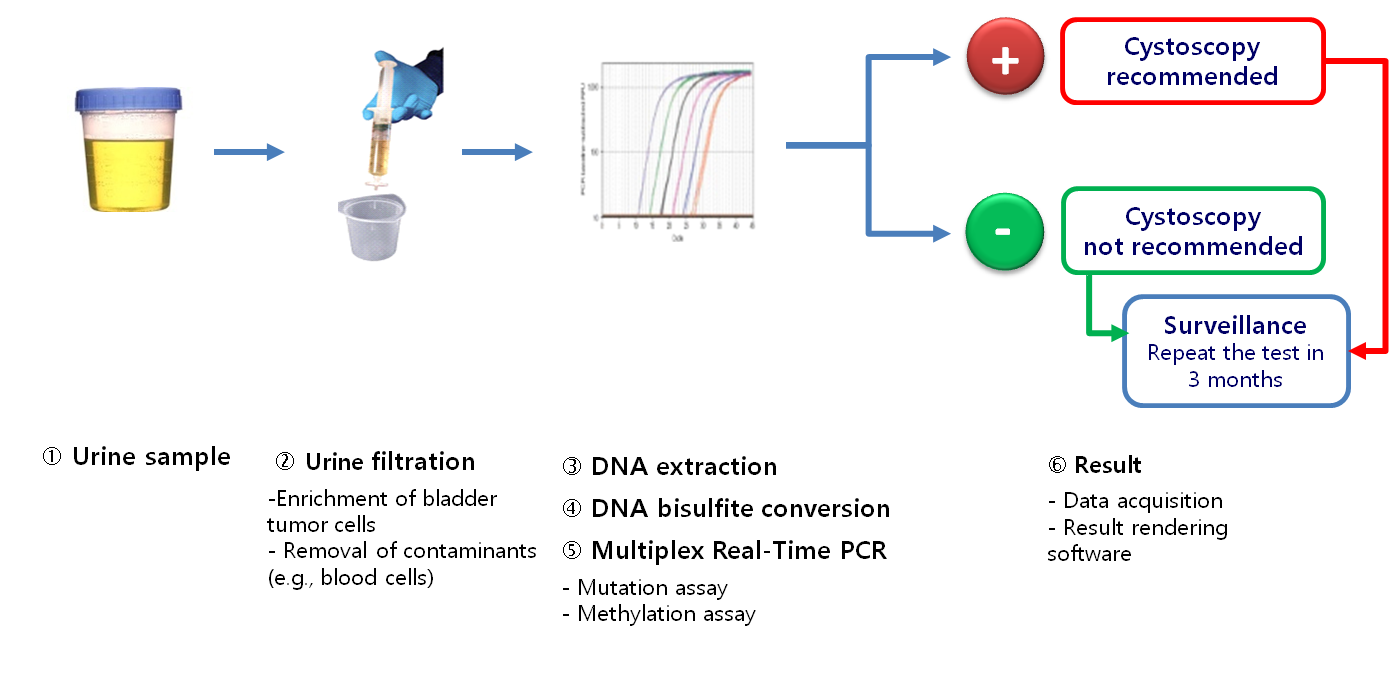
- A simple urine-based test to detect recurrence during the follow-up of non-invasive bladder cancer (NMIBC)
- An integrated genetic and epigenetic analysis
- A sensitive multiplex quantitative real-time PCR
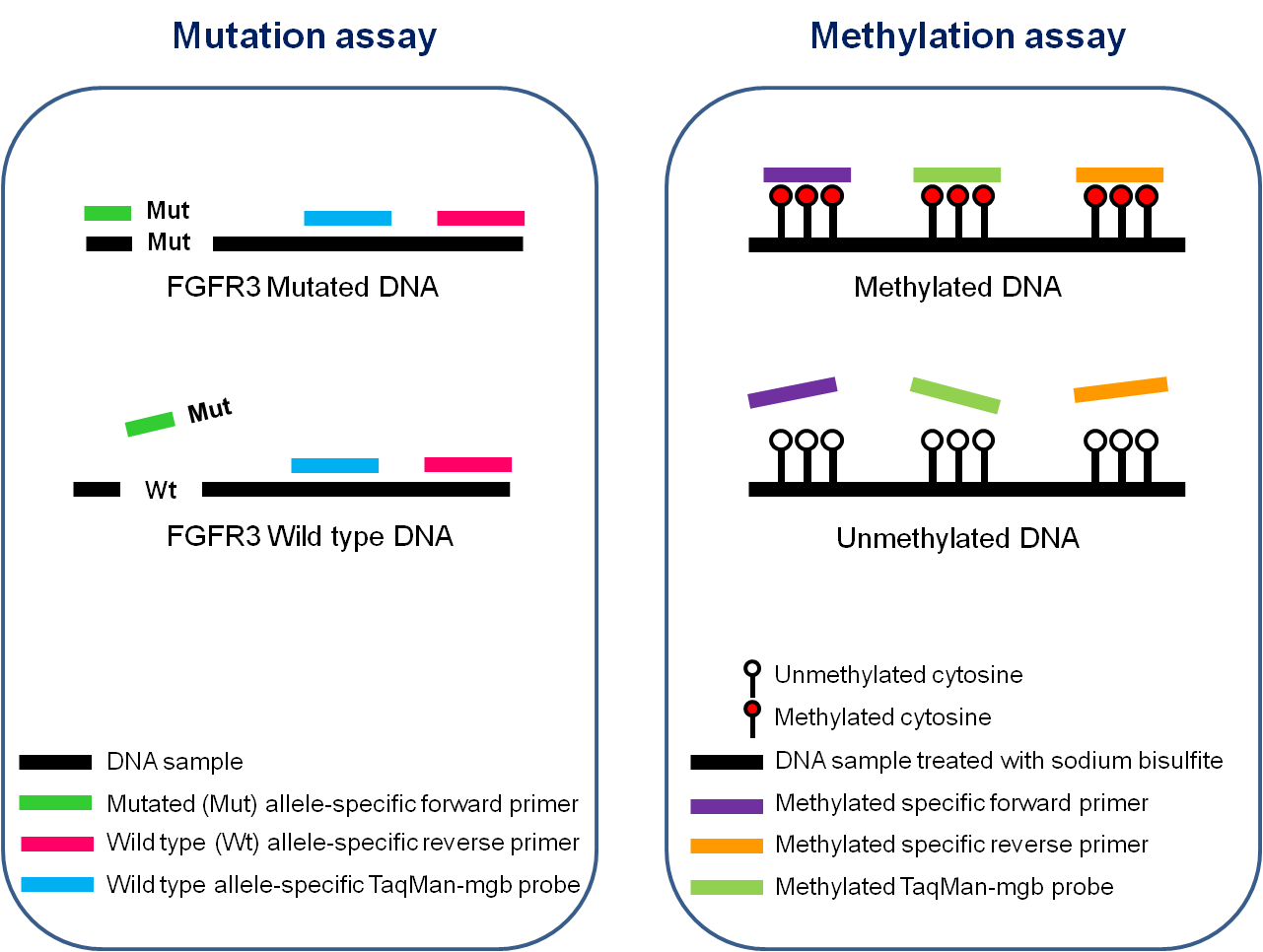
- Mutation assay: Detection of 4 mutations of the FGFR3 gene (G372C, R248C, S249C, Y375C)
- Methylation assay: Quantification of the level of DNA methylation of HS3ST2, SEPTIN9 and SLIT2 genes
- A software for an accurate rendering of results within a 24-hour period
Design of Multiplex PCR assays
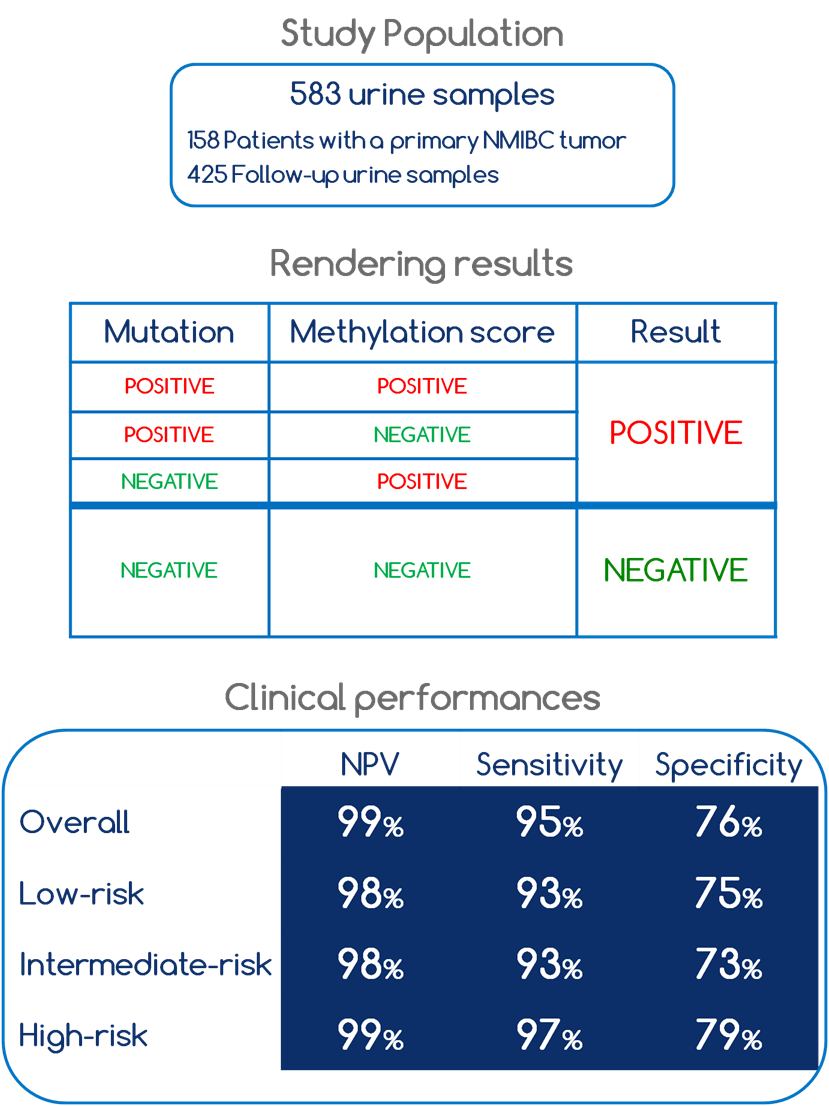
Results of multicenter clinical study
Clinical applications and benefits


EP13306580, 2013: Methods for the surveillance, diagnosis and screening of bladder cancer. November 18, 2014: International extension PCT/EP2014/074892. May 19, 2016: Entering national phase (EU, US, CA, AU, JP)
Publications
Roperch JP and Hennion C. A novel ultra-sensitive method for the detection of FGFR3 mutations in urine of bladder cancer patients - Design of the Urodiag PCR kit for surveillance of patients with non-muscle-invasive bladder cancer (NMIBC). BMC Medical Genetics. 2020 May 24;21(1)112. https://doi.org/10.1186/s12881-020-01050-w
Roperch JP, et al; Promoter hypermethylation of HS3ST2, SEPTIN9 and SLIT2 combined with FGFR3 mutations as a sensitive/specific urinary assay for diagnosis and surveillance in patients with low or high-risk non-muscle-invasive bladder cancer. BMC Cancer. 2016 Sep 1;16:704. https://doi.org/10.1186/s12885-016-2748-5
